| |
|
|
|
|
|
|
|
| |
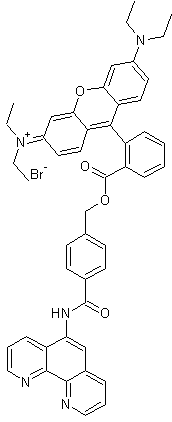
Product Name |
Rhodamine B-[(1,10-phenanthroline-5-yl)-aminocarbonyl]benzyl ester (RPA) |
ME043.1 (1 mg)
ME043.2 (5 mg) |
|
| |
| |  |
|
| |
| |
|
 |
Fe2+ specific fluorescent "sensor" |
|
| |
|
 |
Mitochondria specific |
|
| |
|
 |
Determination of the mitochondrial chelatable iron pool |
|
| |
|
 |
Assessment of: mitochondrial iron uptake |
|
| |
|

| Assessment of alterations of the mitochondrial chelatable iron pool under pathological conditions |
|
| |
|
 |
Assessment of the contribution of mitochondrial chelatable iron to physiological and |
|
| |
|
|
pathological cellular processes |
|
| |
|
 |
Assessment of iron reduction |
|
| |
| | Selective determination of mitochondrial chelatable iron in viable cells with a new fluorescent "iron sensors" RPA and RDA | |
| |
| | Iron is essential for many biological processes, but is also detrimental as it fosters the generation of highly destructive oxygen species. Mitochondrial chelatable iron is considered to contribute to several human diseases. In the past, attempts to determine the intra-mitochondrial pool of chelatable iron were problematic, due to insufficient mitochondrial accumulation or uniform cellular distribution of iron-selective indicators(3). The fluorescent non-toxic "iron sensors" RPA and RDA are the first sensors, which allow to determine this iron pool specifically in single intact mitochondria. They are new tools to assess the function of labile ("redox-active") iron e.g. in processes of cell and tissue injury where free radicals are considered to be involved. | |
| |
| |
Product Information |
|
| |
| |
RDA is used as a selective, high quantum yield fluorescence marker for Fe2+ in biological samples. It has a slightly lower affinity for iron than RPA. The cationic fluorophore of the membrane-permeable compound allows the observation by e.g. fluorescence microscopy and, based on the negative membrane potential of mitochondria, the targeting especially into mitochondria of viable cells. In a cell-free system, RDA fluorescence (λmax 598 nm) is strongly and stoichiometrically (3:1) quenched by Fe2+ ions. |
|
| |
| |  |
|
| |
| |
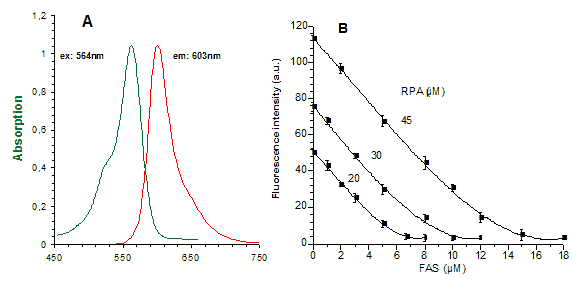
A: |
Absorbance and emission spectra for RPA (20 µM RPA in a "simple buffered solution" (SBS): 2mM ascorbate, 0.15% SDS and 10mM Tris/HCl at pH 8.2) in a cell-free system. |
|
| |
B: |
Effect of Fe2+ on RPA (20-45 µM) fluorescence in "SBS": Fe2+, added as increasing concentrations of ferrous ammonium sulphate/citric acid trisodium salt dehydrate (FAS) from stock solution (1mM) that add. contained 20mM ascorbate, mixed with the medium. Fluorescence (in arbitrary units a.u.) of portions of the medium containing known concentrations of RPA and Fe2+ is determined 2 min later, after complete formation of the RPA/Fe2+ complex. Zero fluorescence is equal to fluorescence of medium without dye. |
|
| |
| |
RDA selectively accumulates in the mitochondria of cells, e.g. of cultured hepatocytes(1,2). Intra-mitochondrial RDA fluorescence is quenched when iron is added to cells in a membrane-permeant form. It increases when the mitochondrial chelatable iron available is experimentally decreased after addition of membrane-permeant transition metal chelators pyridoxal isonicotinoyl hydrazone and 1,10-phenanthroline. This increase of RDA fluorescence allows to specifically quantify mitochondrial chelatable iron in viable cells using ex situ calibration(1). |
|
| |
| |
Application in a cellular system |
|
| |
| |
RDA can be used as described in Lit. 1. Viable cells, cultivated on coverslips for example are incubated with RDA (0.01-10 µM, prepared from stock solutions of 10 µM or 1-5 mM in DMSO) for 10-20 min at 37°C in HBSS ("Hanks balanced salt solution"). Cells are washed subsequently three times with dye-free HBSS. To circumvent RPA binding to the chambers and improve mitochondrial loading, cells should be transferred after RPA loading to a second (indicator-free) Pentz chamber and incubated another 15 min. at 37°C. Cells should now be covered with an apropiate medium (e.g. HBBS or L-15 medium for hepatocytes) at 37°C. Intra-mitochondrial RPA fluorescence is determined using e.g. quantitative laser scanning microscopy. The red fluorescence of RPA is excited at λexc. = 543 nm and collected through a long-pass filter near λexc. = 601 nm. Scanning parameters for quantitative and qualitative measurements depend on the microscope system used for the experiments. The intra-mitochondrial level of chelatable iron can be manipulated 5-10 min after starting of the measurements by addition of FeCl3/8-hydroxyquinoline complex (5-15 µM) or membrane permeant iron chelators, e.g. PIH (pyridoxal isonicotinoyl hydrazone, 2mM) or 1,10-phenanthroline (2 mM). For control measurements, parallel cultures are loaded with the iron-insensitive control RPAC (Rhodamine B-[(phenanthren-9-yl)aminocarbonyl]benzylester) containing the some fluorophore. The same loading conditions should be used as described above. |
|
| |
| |
Ex situ calibration |
|
| |
| |
The ex situ calibration of intra-mitochondrial RDA concentrations is determined by comparing the mitochondrial fluorescence (arbitrary units) after "dequenching" with PIH (2 mM) with the fluorescence of RPA standards (5-80 µM) dissolved in a "mitochondrial medium" (composition see ref. 1). In order to obtain a calibration curve, 100 µl portions of medium with known RPA concentrations are placed on the same coverslips used in cellular experiments. The same focal plane and laser scanning parameters used for the quantitative cellular fluorescence measurements are used in the cell-free ex situ calibrations. |
|
| |
| |
Ex situ calibration of Fe2+ induced quenching of RDA fluorescence |
|
| |
| |
1 ml of "mitochondrial medium" (see ref. 1) is transferred into 1.5 ml tubes, incubated at 37°C. RPA (20-80 µM) is added. Known concentrations of FAS (ferrous ammonium sulphate/citric acid trisodium salt dihydrate) from a freshly prepared stock solution (1 mM) with 20 mM ascorbate are added. The RDA/Fe2+ complex (3:1) is formed during approximately 2 min incubation time. The calibration curve is obtained by measuring the RPA fluorescence with the same focal plane and laser scanning parameters used for the quantitative cellular fluorescence measurements and for the ex situ calibration. |
|
| |
| |
Product Data |
| |
| | chemical name | Rhodamine B-[(1,10-phenanthroline-5-yl)-aminocarbonyl]benzyl ester (RPA)-
| |
| | IUPAC name | 6-(diethylamino)-N,N-diethyl-9-[2-({[4-(1,10-phenanthrolin-5-
ylcarbamoyl)benzyl]oxy}carbonyl)phenyl]-3H-xanthen-3-iminium bromide | |
| | molecular formula | C48H44BrN5O4 | |
| | molecular weight [g/mol] | 834.7981 (79.9045+754.8935) |
|
| | absorption maximum | λmax (log ε) = 562 nm (4.95), 510-535 broad shoulder | |
| | emission maximum | λmax 601 nm | |
| | stability | < 4oC, stored dry and protected from light | |
| | appearance | purple solid | |
| | purity | 97%+ (1H NMR, 500 MHz) | |
| | quenching stoichiometry | RPA/Fe2+: 3:1 [mol/mol] | |
| | in vitro toxicity | non toxic | |
| |
| | Considerations for Use | |
| |
| | The product is used as a selective, high quantum yield fluorescence marker for Fe2+ in biological samples, especially in mitochondria of viable cells. RDA has a slightly lower affinity to iron compared to RPA. Measurements can be performed by fluorescence spectroscopy, fluorescence plate readers, FACS, video microscopy and laser scanning microscopy. 1-5 mM stock solutions of RDA in DMSO can be prepared and aliquots should kept at -20°C. When stored properly at -20°C, the solutions can be used for at least 2 - 3 months. | |
| |
| | Literature | |
| |
| | 1. Selective determination of mitochondrial chelatable iron in viable cells with a new fluorescent sensor. F. Petrat et. al. Biochem. J. (2002) 362, 137-147
2. Cold-induced apoptosis of hepatocytes: mitochondrial permeability transition triggered by nonmitochondrial chelatable iron; U. Rauen et al. Free Radical Biology & Medicine, Vol. 35, No. 12, pp. 1664-1678, 2003
3. The chelatable iron pool in living cells: A methodically defined quantity. F. Petrat et. al. Biol. Chem., Vol. 383, pp. 489-502, 2002
4. Assessment of chelatable mitochondrial iron by using mitochondrion-selective fluorescent iron indicators with different iron-binding affinities. U. Rauen et al. ChemBioChem 2007, 8, 341-352
5. Oxidative inactivation of mitochondrial Aconitase results in iron and hydrogen peroxide-mediated neurotoxicity in rat primary mesencephalic cultures. David Cantu et al. PlosOne, September 2009, Vol 4, Issue 9, p 1-9
6. Tryparedoxin peroxidase-deficiency commits trypanosomes to ferroptosis-type cell death. M. Bogacz et al. Cell Biology, July 2018, eLife 2018. 7:e37503 doi: 10.7554/eLife.37503
7. Mitochondrial ferritin limits oxidative damage regulating mitochondrial iron availability: hypothesis for a protective role in Friedreich ataxia. Alessandro Campanella et. Al. Hum. Mol. Genet. 2009, Jan 1. 18(1): 1-11
8. Uptake of Non-Transferrin Iron by Erythroid Cells. Eugenia Prus et al. Anemia. 2011. 2011: 945289
9. Chelation of mitochondrial iron prevents seizure-induced mitochondrial dysfunction and neuronal injury. Li-Ping Liang et al. Journal of Neuroscience 5 November 2008, 28 (45) 11550-11556
10. Reduction in mitochondrial iron alleviates cardiac damage during injury. Hsiang-Chun Chang et al. EMBO Molecular Medicine: 8 (3) (2016), 247-267
11. Cardiotoxicity of doxorubicin is mediated through mitochondrial iron accumulation. Yoshihiko Ichikawa et al. Cardiology. J Clin Invest. 2014 Feb 3. 124(2): 617-630.
12. Iron chelators for treating and preventing cell death and organ damage following an ischemic event. Hossein Ardehali et al. 2016. US20170014397A1, US patent.
13. Mitochondrial iron and energetic dysfunction distinguish fibroblasts and induced neurons from pantothenate kinase-associated neurodegeneration patients. Paolo Santambrogio et al. Neurobiology of Disease, Vol. 81, September 2015, pp 144-153
14. Shawn, the Drosophila homolog of SLC25A39/40, is a mitochondrial carrier that promotes neuronal survival. Jan R. Slabbaert et al. The Journal of Neuroscience 10 February 2016, 36 (6) 1914-1929.
15. Iron regulatory protein deficiency compromises mitochondrial function in murine embryonic fibroblasts. Huihui Li et. al. Nature, Scientific Reports volume 8, Article number: 5118 (2018)
16. Iron-chelator complexes as iron sources for early developing humanbovine erythroid precursors. J.M. Leimberg et al., Translational Research 2008, 151:88-96
17. Friedreich's Ataxia, No Changes in Mitochondrial Labile Iron in bovine Lymphoblasts and Fibroblasts. F. Petrat et al., J. biology. Chem. 280, 8, February 25, pp. 6701-6708, 2005
18. Designer Aminoglycosides That Selectively Inhibit Cytoplasmic Rather than Mitochondrial Ribosomes Show Decreased Ototoxicity. Timor Baasov et al., J. biol. Chem. 289/4, pp. 2318-2330. January 24, 2014
| |
| |
| | Ordering Information | |
| |
| |
| |
Cat. No. |
| |
Product Name |
| |
Quantity |
| |
Price |
|
| |
ME043.1 |
|
Rhodamine B-[(1,10-phenanthroline-5-yl)-aminocarbonyl]benzyl ester (RPA) |
|
1 mg |
|
325,- € |
|
| |
ME043.2 |
|
Rhodamine B-[(1,10-phenanthroline-5-yl)-aminocarbonyl]benzyl ester (RPA) |
|
5 mg |
|
975,- € |
|
| |
|
* For bulk package size please inquire. Prices do not contain shipping costs, please inquire. |
|
| |