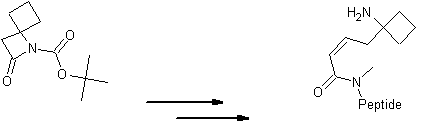
| Volume 5 | ||||||||||||||||||||
| New spirocyclic β-Lactams | ||||||||||||||||||||
| The Squarix Newsletter provides you with information about our innovative building blocks, reactive intermediates and screening compounds from our Discovery Chemicals portfolio. | ||||||||||||||||||||
| |
||||||||||||||||||||
| In this issue we present new spirocyclic β-Lactams especially designed for stereocontrolled synthesis of peptidomimetics. | ||||||||||||||||||||
| Featured Products | ||||||||||||||||||||
|
||||||||||||||||||||
 |
 |
 |
||||||||||||||||||
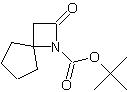
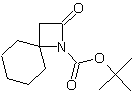
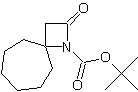
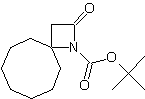
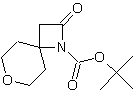
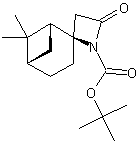
| LA066 | LA075 | LA078 | ||||||||||||||||||
| These compounds are derived from natural and unnatural amino acids and are not commercially available from other manufacturers. | ||||||||||||||||||||
| See our website for the full line of building blocks or download the Discovery Chemicals catalogue. | ||||||||||||||||||||
| Please find also some selected references from the literature describing interesting applications of spirocyclic β-Lactams. Various dipeptidomimetic N-terminals were synthesized using the key building block LA064 (Peschke et al.). Palomo et al. reported an efficient syntheses of short peptide segments using the spirocyclic β-Lactams LA064, LA048 and LA049 as starting material: | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| References | ||||||||||||||||||||
|
||||||||||||||||||||
| Discovery Chemicals | |
| Custom Synthesis | |
| Call us for a quote: +49-2365-20400-0 |
|
| Squarix GmbH Elbestraße 10 D-45768 Marl Germany |
|
| fon: +49-2365-20400-0 fax: +49-2365-20400-60 web: www.squarix.de e-mail: info@squarix.de |
|
| subscribe to Squarix newsletter | |
| unsubscribe from Squarix newsletter | |
 |
|
| Download catalogue | |
| [PDF] | |
modification or cancellation. Do not hesitate to use this opportunity by sending a message to info@squarix.de